And of course you could redo this calculation to find the volume of 1 mole of an ideal gas at room temperature and pressure or any other temperature and pressure.
Room temperature and pressure volume of gas.
Temperature can be measured using the celsius and kelvin scales.
Satp standard ambient temperature and pressure is also used in chemistry as a reference.
Before 1918 many professionals and scientists using the metric system of units defined the standard reference conditions of temperature and pressure for expressing gas volumes as being 15 c 288 15 k.
Finding the relative formula mass of a gas from its density.
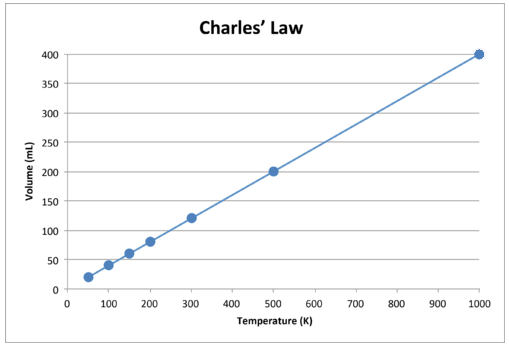
Charles s law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant.
Gas pressure increases with temperature.
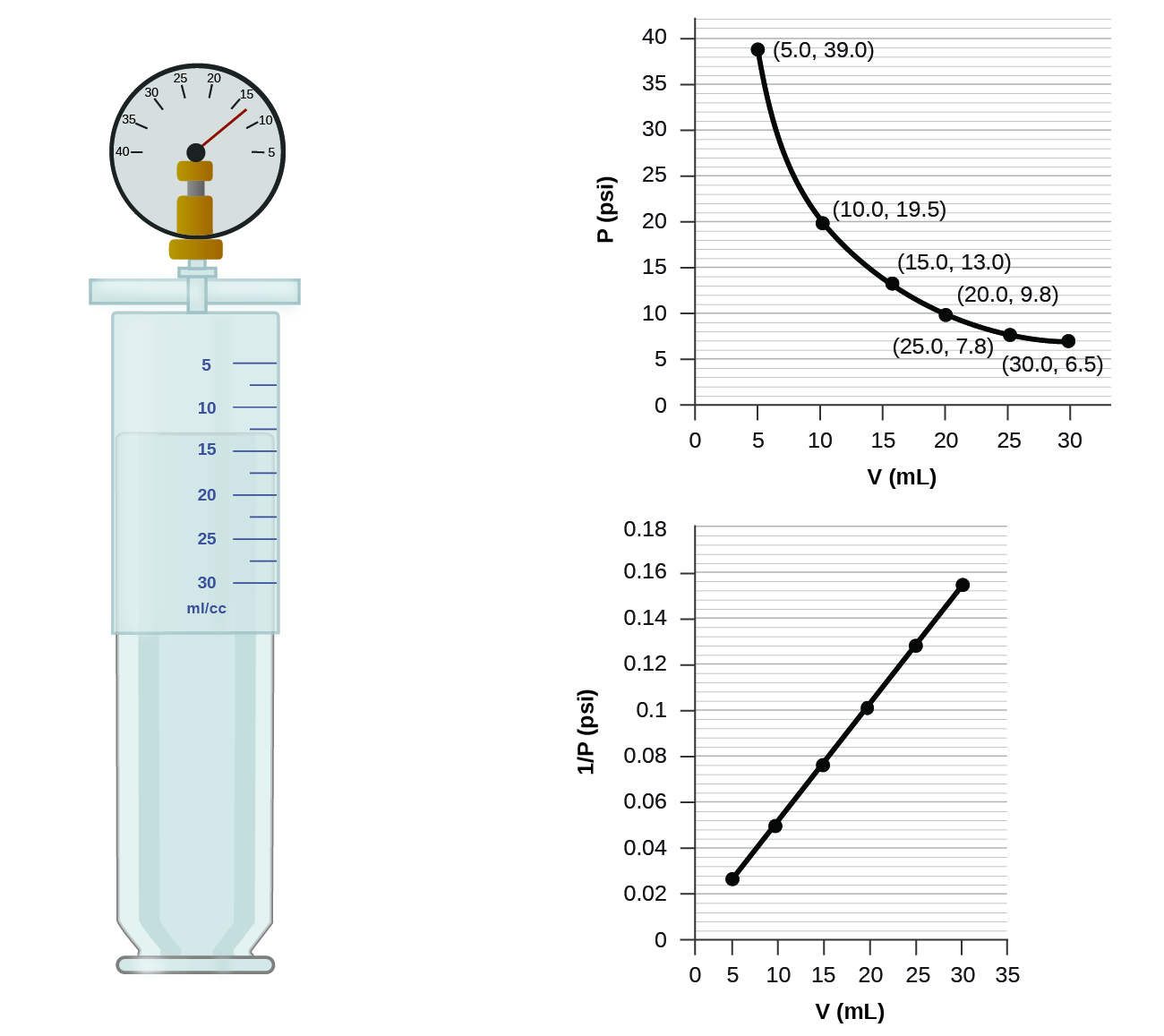
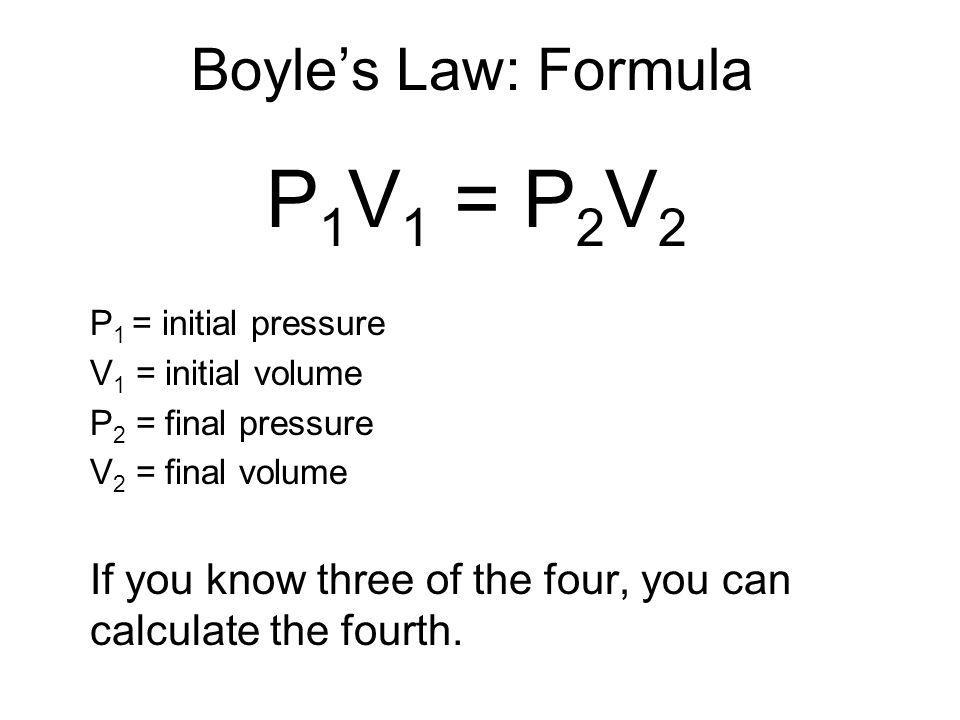
As the volume goes up the temperature also goes up and vice versa.
One mole of any gas has a volume of 24 dm 3 or 24 000 cm 3 at rtp room temperature and pressure.
French physicist jacques charles 1746 1823 studied the effect of temperature on the volume of a gas at constant pressure.
Charles law gives the relationship between volume and temperature if pressure and amount of gas are held constant.
The absolute temperature is temperature measured with the kelvin scale.
59 00 f and 101 325 kpa 1 00 atm.
760 torr during those same years the most commonly used standard reference conditions for people using the imperial or u s.
Easily calculate the pressure volume temperature or quantity in moles of a gas using this combined gas law calculator boyle s law calculator charles s law calculator avogadro s law calculator and gay lussac s law calculator in one supports a variety of input metrics such as celsius fahrenheit kelvin pascals bars atmospheres and volume in both metric and.
The molar volume of an ideal gas is therefore 22 4 dm 3 at stp.
This is about as tricky as it gets using the ideal gas.
At these conditions the volume of 1 mol of a gas is 24 4651 liters.
Satp standard ambient temperature and pressure is a reference with temperature of 25 o c 298 15 k and pressure of 101 325 kpa.
Equations explain the relationship between pressure temperature and volume in gases.
Ideal gas law calculator.
Ideal gas law relation between the pressure volume amount and temperature of a gas under conditions derived by combination of the simple gas laws standard conditions of temperature and pressure stp 273 15 k 0 c and 1 atm 101 325 kpa standard molar volume volume of 1 mole of gas at stp approximately 22 4 l for gases behaving ideally.
The temperature volume law jacques charles 1746 1823 this law states that the volume of a given amount of gas held at constant pressure is directly proportional to the kelvin temperature.
This volume is called the molar volume of a gas.
V t c.
2 if the volume of a container is decreased the temperature decreases.
Satp standard ambient temperature and pressure.
This means that the volume of a gas is directly proportional to its temperature.